Textbook Question
Give the symbol for (b) a beta particle.
424
views

 Verified step by step guidance
Verified step by step guidance
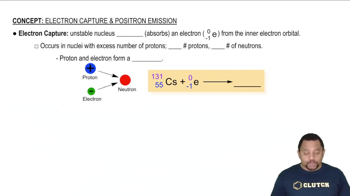


Give the symbol for (b) a beta particle.
Give the symbol for (c) a positron.
Write balanced nuclear equations for the following processes: (a) rubidium-90 undergoes beta emission.
Write balanced nuclear equations for the following processes: (c) krypton-76 undergoes positron emission.
Write balanced nuclear equations for the following processes: (d) radium-226 emits alpha radiation.
Write balanced nuclear equations for the following transformations: (a) bismuth-213 undergoes alpha decay.