Figure 7.4 shows the radial probability distribution functions for the 2s orbitals and 2p orbitals. (a) Which orbital, 2s or 2p, has more electron density close to the nucleus?
Ch.7 - Periodic Properties of the Elements

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 7, Problem 81b
(b) Repeat these calculations using Slater’s rules.
 Verified step by step guidance
Verified step by step guidance1
Identify the electron configuration of the element in question. Determine the number of electrons in each shell and subshell.
Apply Slater's rules to calculate the shielding constant (\( \sigma \)) for each electron. Remember that electrons in the same group, inner shells, and different shells contribute differently to the shielding.
Calculate the effective nuclear charge (\( Z_{\text{eff}} \)) using the formula: \( Z_{\text{eff}} = Z - \sigma \), where \( Z \) is the atomic number and \( \sigma \) is the total shielding constant.
Consider the contributions of different electron groups: Electrons in the same shell, electrons in the \( n-1 \) shell, and electrons in shells \( n-2 \) or lower, each with their respective shielding values.
Sum up the contributions from all electrons to find the total shielding constant, and use it to determine the effective nuclear charge.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Slater's Rules
Slater's Rules are a set of guidelines used to estimate the shielding effect of electrons in multi-electron atoms. They provide a systematic way to calculate the effective nuclear charge (Z_eff) experienced by an electron, taking into account the contributions of other electrons. By applying these rules, one can determine how much an electron is shielded from the full positive charge of the nucleus, which is crucial for understanding atomic structure and electron configurations.
Recommended video:
Guided course
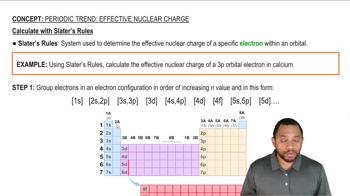
Effective Nuclear Charge Calculation with Slater's Rules
Effective Nuclear Charge (Z_eff)
Effective Nuclear Charge (Z_eff) is the net positive charge experienced by an electron in a multi-electron atom. It is calculated by subtracting the shielding effect of other electrons from the actual nuclear charge. Z_eff influences various atomic properties, including ionization energy and atomic size, as it determines how strongly an electron is held by the nucleus. Understanding Z_eff is essential for predicting the behavior of atoms in chemical reactions.
Recommended video:
Guided course
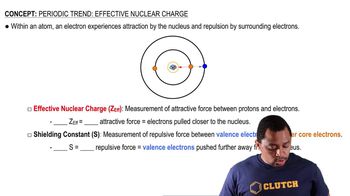
Effective Nuclear Charge
Shielding Effect
The shielding effect refers to the phenomenon where inner-shell electrons partially block the attractive force of the nucleus on outer-shell electrons. This results in outer electrons experiencing a reduced effective nuclear charge. The extent of shielding varies depending on the electron configuration and the arrangement of electrons in an atom. Recognizing the shielding effect is vital for accurately calculating Z_eff and understanding trends in the periodic table.
Recommended video:
Guided course

Photoelectric Effect
Related Practice
Textbook Question
843
views
Textbook Question
Figure 7.4 shows the radial probability distribution functions for the 2s orbitals and 2p orbitals. (b) How would you modify Slater's rules to adjust for the difference in electronic penetration of the nucleus for the 2s and 2p orbitals?
2114
views
Textbook Question
(a) If the core electrons were totally effective at screening the valence electrons and the valence electrons provided no screening for each other, what would be the effective nuclear charge acting on the 3s and 3p valence electrons in P?
1082
views
Textbook Question
(c) Detailed calculations indicate that the effective nuclear charge is 5.6+ for the 3s electrons and 4.9+ for the 3p electrons. Why are the values for the 3s and 3p electrons different?
Textbook Question
(d) If you remove a single electron from a P atom, which orbital will it come from?
1056
views
