(a) When chlorine atoms react with atmospheric ozone, what are the products of the reaction?

Nitrogen oxides like NO2 and NO are a significant source of acid rain. For each of these molecules, write an equation that shows how an acid is formed from the reaction with water.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Acid-Base Reactions

Formation of Nitric Acid
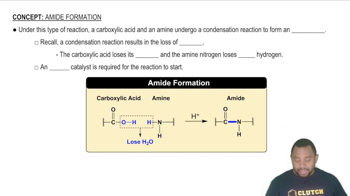
Formation of Nitrous Acid

(b) Based on average bond enthalpies, would you expect a photon capable of dissociating a C-Cl bond to have sufficient energy to dissociate a C-Br bond?
(b) If a limestone sculpture were treated to form a surface layer of calcium sulfate, would this help to slow down the effects of acid rain? Explain.
Alcohol-based fuels for automobiles lead to the production of formaldehyde (CH2O) in exhaust gases. Formaldehyde undergoes photodissociation, which contributes to photo- chemical smog: CH2O + hn ¡ CHO + H The maximum wavelength of light that can cause this reaction is 335 nm. (b) What is the maximum strength of a bond, in kJ/mol, that can be broken by absorption of a photon of 335-nm light?
Alcohol-based fuels for automobiles lead to the production of formaldehyde (CH2O) in exhaust gases. Formaldehyde undergoes photodissociation, which contributes to photo- chemical smog: CH2O + hn ¡ CHO + H The maximum wavelength of light that can cause this reac- tion is 335 nm. (d) Write out the formaldehyde photodis- sociation reaction, showing Lewis-dot structures.
