Textbook Question
Predict the anode, cathode, and overall cell reactions when an aqueous solution of each of the following salts is electrolyzed in a cell having inert electrodes. (c) LiOH
455
views

 Verified step by step guidance
Verified step by step guidance

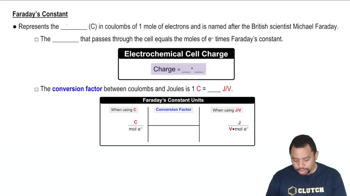
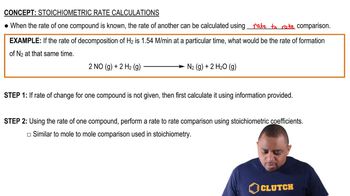
Predict the anode, cathode, and overall cell reactions when an aqueous solution of each of the following salts is electrolyzed in a cell having inert electrodes. (c) LiOH
In order to charge a lead storage battery (Section 19.10) 500.0 g of PbSO4(s) must be converted into PbO2(s) and Pb(s). (a) Does the reaction represent an electrolytic or galvanic cell?