Textbook Question
How many hours are required to produce 1.00 * 103 kg of sodium by the electrolysis of molten NaCl with a constant current of 3.00 * 104 A? How many liters of Cl2 at STP will be obtained as a by-product?
196
views

 Verified step by step guidance
Verified step by step guidance

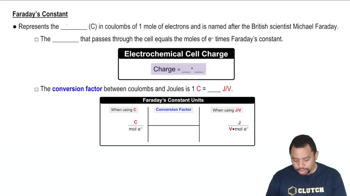

In order to charge a lead storage battery (Section 19.10) 500.0 g of PbSO4(s) must be converted into PbO2(s) and Pb(s). (a) Does the reaction represent an electrolytic or galvanic cell?
In order to charge a lead storage battery (Section 19.10) 500.0 g of PbSO4(s) must be converted into PbO2(s) and Pb(s). (b) How many coulombs of electrical charge are needed?
In order to charge a lead storage battery (Section 19.10) 500.0 g of PbSO4(s) must be converted into PbO2(s) and Pb(s). (c) If a current of 500 A is used, how long will it take?