Textbook Question
Predict the anode, cathode, and overall cell reactions when an aqueous solution of each of the following salts is electrolyzed in a cell having inert electrodes. (a) Ag2SO4
1276
views

 Verified step by step guidance
Verified step by step guidance

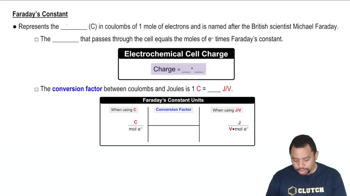

In order to charge a lead storage battery (Section 19.10) 500.0 g of PbSO4(s) must be converted into PbO2(s) and Pb(s). (a) Does the reaction represent an electrolytic or galvanic cell?
In order to charge a lead storage battery (Section 19.10) 500.0 g of PbSO4(s) must be converted into PbO2(s) and Pb(s). (b) How many coulombs of electrical charge are needed?