Textbook Question
What are the molecular weights of the following herbicides? (b) C15H22ClNO2 (metolachlor, pre-emergent herbicide)
387
views

 Verified step by step guidance
Verified step by step guidance

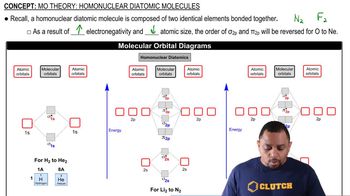

What are the molecular weights of the following herbicides? (b) C15H22ClNO2 (metolachlor, pre-emergent herbicide)
What are the molecular weights of the following herbicides? (c) C8H6Cl2O3 (dicamba, effective on broadleaf plants
How many grams are in a mole of each of the following substances? (a) Ti
How many grams are in a mole of each of the following substances? (c) Hg
How many grams are in a mole of each of the following substances? (d) H2O
How many moles of ions are in 27.5 g of MgCl2