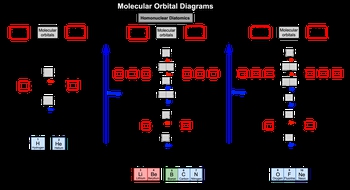
A whole nuclear diatomic molecule consists of two identical elements bonded together, such as nitrogen (N2) or fluorine (F2). The molecular orbital theory explains how the arrangement of electrons in these molecules changes based on the elements involved. As electronegativity increases and atomic size decreases, the order of molecular orbitals for elements from oxygen to neon is reversed.
In molecular orbital diagrams, we first consider hydrogen (H2) and helium (He2). Both hydrogen and helium have their valence electrons in the 1s orbital, which is the first period of the periodic table. The bonding molecular orbital is represented as σ1s, while the antibonding molecular orbital is denoted as σ1s*.
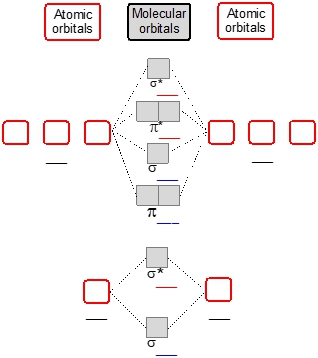
When examining diatomic lithium (Li2) through diatomic nitrogen (N22s, followed by the antibonding molecular orbital σ2s* and then the 2p orbitals. For the 2p orbitals, the order of filling is π2p, σ2p, π2p*, and σ2p*.
As we move from diatomic oxygen (O2) to diatomic neon (Ne2), the order of the molecular orbitals changes due to the increased electronegativity and decreased atomic size. In this case, π2p moves to a higher energy level, while σ2p moves to a lower energy level. This shift is crucial for understanding the stability and bonding characteristics of these molecules.
Overall, the arrangement of molecular orbitals varies depending on the period of the elements involved, influencing the properties and behaviors of homonuclear diatomic molecules. Understanding these concepts is essential for predicting molecular stability and reactivity in various chemical contexts.