Electron Configuration
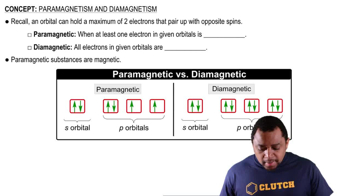
Electron configuration describes the distribution of electrons in an atom's orbitals. For vanadium (V), with an atomic number of 23, the electron configuration is [Ar] 3d³ 4s². Understanding this configuration is crucial to determine how many unpaired electrons are present, which directly relates to the paramagnetic property.

 Verified step by step guidance
Verified step by step guidance