Textbook Question
Convert each of the following line-angle formulas to a condensed structural formula and give its IUPAC name:
a.
815
views

 Timberlake 13th Edition
Timberlake 13th Edition Ch.11 Introduction to Organic Chemistry: Hydrocarbons
Ch.11 Introduction to Organic Chemistry: Hydrocarbons Problem 48a
Problem 48a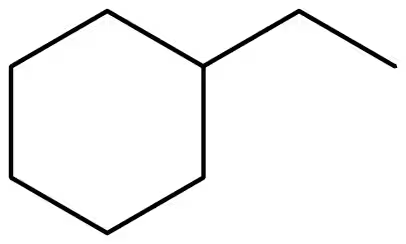
 Verified step by step guidance
Verified step by step guidance



Convert each of the following line-angle formulas to a condensed structural formula and give its IUPAC name:
a.
Convert each of the following line-angle formulas to a condensed structural formula and give its IUPAC name:
b.
Give the IUPAC name for each of the following:
c.
Give the IUPAC name for each of the following:
c.
Give the IUPAC name (including cis or trans, if needed) for each of the following:
a.
Give the IUPAC name (including cis or trans, if needed) for each of the following:
c.