Textbook Question
Draw the condensed structural formula for the ester formed when each of the following reacts with ethyl alcohol:
b. propionic acid
585
views

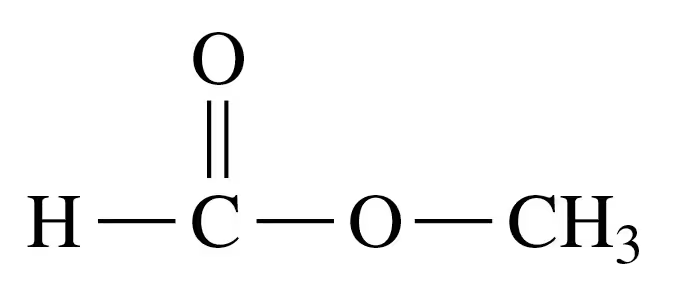
 Verified step by step guidance
Verified step by step guidance

Draw the condensed structural formula for the ester formed when each of the following reacts with ethyl alcohol:
b. propionic acid
Draw the condensed structural or line-angle formula for the ester formed in each of the following reactions:
b.
Draw the condensed structural or line-angle formula for the ester formed in each of the following reactions:
b.
Draw the condensed structural formulas for a and b and line-angle formulas for c and d:
b. butyl formate
Draw the condensed structural formulas for a and b and line-angle formulas for c and d:
d. methyl propanoate
Draw the condensed structural formulas for a and b and line-angle formulas for c and d:
c. propyl benzoate