Using each of the following electron arrangements, give the formulas for the cation and anion that form, the formula for the compound they form, and its name.
Ch.6 Ionic and Molecular Compounds

Timberlake13th EditionChemistry: An Introduction to General, Organic, and Biological ChemistryISBN: 9780134421353Not the one you use?Change textbook
Chapter 6, Problem 104a
Match each of the formulas (a to c) with the correct diagram (1 to 3) of its shape, and name the shape; indicate if each molecule is polar or nonpolar.
<IMAGE>
a. PBr3
 Verified step by step guidance
Verified step by step guidance1
Step 1: Determine the central atom in the molecule PBr₃. Phosphorus (P) is the central atom because it is less electronegative than bromine (Br) and can form multiple bonds.
Step 2: Count the total number of valence electrons in the molecule. Phosphorus has 5 valence electrons, and each bromine atom has 7 valence electrons. Add these together: 5 + (7 × 3) = 26 valence electrons.
Step 3: Draw the Lewis structure for PBr₃. Place phosphorus in the center and connect it to each bromine atom with a single bond. Distribute the remaining electrons to satisfy the octet rule for each atom, starting with the bromine atoms. Any leftover electrons should be placed as a lone pair on the phosphorus atom.
Step 4: Determine the molecular geometry using VSEPR (Valence Shell Electron Pair Repulsion) theory. Phosphorus has three bonding pairs and one lone pair of electrons. This arrangement corresponds to a trigonal pyramidal shape.
Step 5: Assess the polarity of the molecule. The P–Br bonds are polar due to the difference in electronegativity between phosphorus and bromine. However, because the molecule has a trigonal pyramidal shape, the dipole moments do not cancel out, making PBr₃ a polar molecule.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
10mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It is determined by the number of bonding pairs and lone pairs of electrons around the central atom, which influences the shape of the molecule. Understanding molecular geometry is crucial for predicting the physical and chemical properties of substances, including polarity.
Recommended video:
Guided course
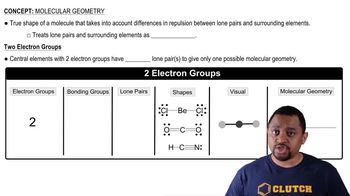
Molecular Geometry (Simplified) Concept 1
Polarity of Molecules
Polarity in molecules arises from the distribution of electrical charge, which is influenced by the electronegativity of the atoms involved and the molecular geometry. A molecule is polar if it has a net dipole moment due to an uneven distribution of charge, while nonpolar molecules have symmetrical charge distributions. Identifying polarity is essential for understanding intermolecular interactions and solubility.
Recommended video:
Guided course

Molecular Polarity (Simplified) Concept 1
VSEPR Theory
Valence Shell Electron Pair Repulsion (VSEPR) theory is a model used to predict the shape of molecules based on the repulsion between electron pairs in the valence shell of the central atom. According to VSEPR, electron pairs will arrange themselves to minimize repulsion, leading to specific molecular shapes. This theory is fundamental for determining the geometry and polarity of molecules like PBr₃.
Recommended video:
Guided course

Atomic Theory
Related Practice
Textbook Question
853
views
Textbook Question
State the number of valence electrons, bonding pairs, and lone pairs in each of the following Lewis structures:
c.
1678
views
Textbook Question
Match each of the Lewis structures (a to c) with the correct diagram (1 to 3) of its shape, and name the shape; indicate if each molecule is polar or nonpolar. Assume X and Y are nonmetals and all bonds are polar covalent.
<IMAGE>
c.
1744
views
Textbook Question
Consider the following bonds: Ca and O, C and O, K and O, O and O, and N and O.
d. Arrange the covalent bonds in order of decreasing polarity.
1544
views
Textbook Question
Consider an ion with the symbol X2+ formed from a representative element.
b. What is the Lewis symbol of the element?
1006
views
Textbook Question
Consider an ion with the symbol X2+ formed from a representative element.
c. If X is in Period 3, what is the element?
968
views
