Textbook Question
A container was found in the home of the victim that contained 120 g of ethylene glycol in 450 g of liquid. What was the percentage of ethylene glycol? Express your answer to the ones place.
1502
views

 Verified step by step guidance
Verified step by step guidance

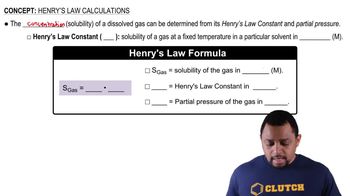

A container was found in the home of the victim that contained 120 g of ethylene glycol in 450 g of liquid. What was the percentage of ethylene glycol? Express your answer to the ones place.
If the toxic quantity is 1.5 g of ethylene glycol per 1000 g of body mass, what percentage of ethylene glycol is fatal?
A bag of gumdrops contains 16 orange gumdrops, 8 yellow gumdrops, and 16 black gumdrops.
a. What is the percentage of yellow gumdrops? Express your answer to the ones place.