Textbook Question
Using TABLE 10.3, identify the stronger acid in each of the following pairs:
a. HBr or HNO2
885
views

 Verified step by step guidance
Verified step by step guidance


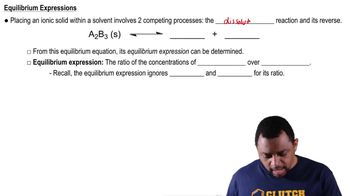
Using TABLE 10.3, identify the stronger acid in each of the following pairs:
a. HBr or HNO2
Using TABLE 10.3, identify the stronger acid in each of the following pairs:
b. H3PO4 or HSO4-
What is meant by the term reversible reaction?
If a base is added to pure water, why does the [H3O+] decrease?
Calculate the [H3O+] of each aqueous solution with the following [OH-]:
d. bile, 2.5 × 10-6 M
Calculate the [H3O+] of each aqueous solution with the following [OH-]:
a. baking soda, 1.0 × 10-6 M