Draw the line-angle formula for each of the following compounds:
c. 4-ethyltoluene

 Timberlake 13th Edition
Timberlake 13th Edition Ch.11 Introduction to Organic Chemistry: Hydrocarbons
Ch.11 Introduction to Organic Chemistry: Hydrocarbons Problem 41a
Problem 41a Verified step by step guidance
Verified step by step guidance


Draw the line-angle formula for each of the following compounds:
c. 4-ethyltoluene
Draw the line-angle formula for each of the following compounds:
c. 1,2,4-trichlorobenzene
Write the balanced chemical equation for the complete combustion of each of the following hydrocarbons found in gasoline:
c. 3-ethyltoluene
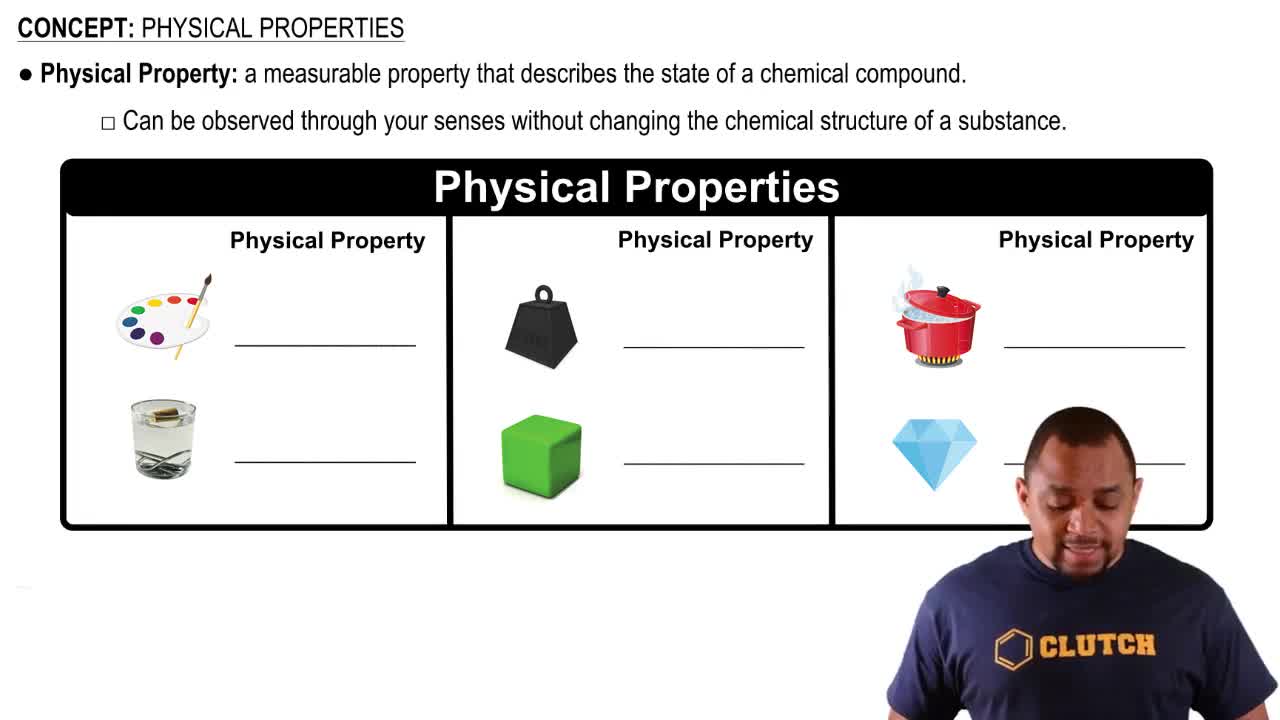
Match the following physical and chemical properties with potassium chloride, KCl, used in salt substitutes, or butane, C4H10 used in lighters:
<IMAGE>
e. is a gas at room temperature
Match the following physical and chemical properties with octane, C8H18 found in gasoline, or magnesium sulfate, MgSO4 also called Epsom salts:
a. contains only covalent bonds
Match the following physical and chemical properties with octane, C8H18 found in gasoline, or magnesium sulfate, MgSO4 also called Epsom salts:
d. is a liquid at room temperature