Textbook Question
A compound with the formula C4H8O is synthesized from 2-methyl-1-propanol and oxidizes easily to give a carboxylic acid. Draw the condensed structural formula and give the IUPAC name for the compound.
885
views

 Timberlake 13th Edition
Timberlake 13th Edition Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones
Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones Problem 67
Problem 67 Verified step by step guidance
Verified step by step guidance


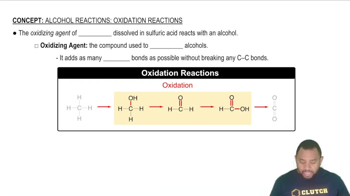
A compound with the formula C4H8O is synthesized from 2-methyl-1-propanol and oxidizes easily to give a carboxylic acid. Draw the condensed structural formula and give the IUPAC name for the compound.
A compound with the formula C5H10O oxidizes to give 3-pentanone. Draw the condensed structural formula and give the IUPAC name for the compound.
Compound X is a secondary alcohol whose formula is C3H8O. When compound X is heated with strong acid, it dehydrates to form compound Y (C3H6). When compound X is oxidized, compound Z (C3H6O) forms, which cannot be oxidized further. Draw the condensed structural formulas and give the IUPAC names for compounds X, Y, and Z.