Which compound in each of the following pairs would be more soluble in water? Explain.
b. propanone or 3-hexanone

 Timberlake 13th Edition
Timberlake 13th Edition Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones
Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones Problem 27b
Problem 27b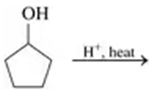
 Verified step by step guidance
Verified step by step guidance


Which compound in each of the following pairs would be more soluble in water? Explain.
b. propanone or 3-hexanone
Which compound in each of the following pairs would be more soluble in water? Explain.
c. butanal or hexanal
Write the balanced chemical equation for the complete combustion of each of the following compounds:
b. 3-hexanol
Draw the condensed structural or line-angle formula for the alkene produced by each of the following dehydration reactions:
b.
Draw the condensed structural or line-angle formula for the aldehyde or ketone formed when each of the following alcohols is oxidized [O] (if no reaction, write none):
d.
Draw the condensed structural formulas for the aldehyde and carboxylic acid produced when each of the following is oxidized:
c. 3-chloro-1-propanol