Textbook Question
Draw the condensed structural formulas for a and b and line-angle formulas for c and d:
c. 3-bromopentanoic acid
566
views

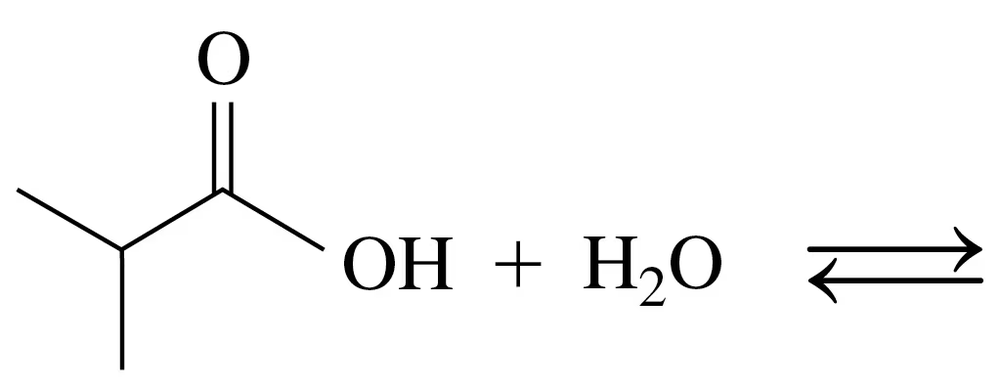
 Verified step by step guidance
Verified step by step guidance


Draw the condensed structural formulas for a and b and line-angle formulas for c and d:
c. 3-bromopentanoic acid
Draw the condensed structural formulas for a and b and line-angle formulas for c and d:
a. pentyl formate
Draw the condensed structural or line-angle formulas for the products of the following:
c.
Draw the condensed structural or line-angle formulas for the products of the following:
d.
Draw the condensed structural or line-angle formulas for the products of the following:
c.
Draw the condensed structural or line-angle formulas for the products of the following:
a.