Peptides from sweet potato with antioxidant properties have the following sequence of amino acids. Draw the structure for each peptide and write the one-letter abbreviations.
b. Asn–Tyr–Asp–Glu–Tyr

 Verified step by step guidance
Verified step by step guidance

Peptides from sweet potato with antioxidant properties have the following sequence of amino acids. Draw the structure for each peptide and write the one-letter abbreviations.
b. Asn–Tyr–Asp–Glu–Tyr
Explain why each of the following pairs are complementary proteins:
a. beans and oats
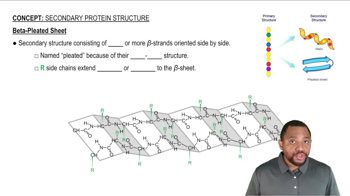
What happens when a primary structure forms a secondary structure?
What type of interaction would you expect between the R groups of the following amino acids in a tertiary structure?
c. serine and aspartate
What type of interaction would you expect between the R groups of the following amino acids in a quaternary structure?
a. phenylalanine and isoleucine
What type of interaction would you expect between the R groups of the following amino acids in a quaternary structure?
d. alanine and proline