Textbook Question
What component in a nucleic acid determines the 5' free end?
535
views

 Verified step by step guidance
Verified step by step guidance
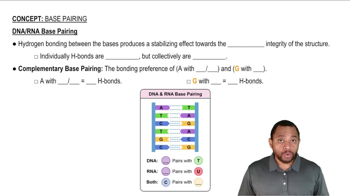

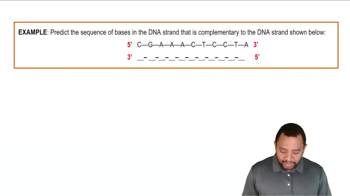
What component in a nucleic acid determines the 5' free end?
Draw the condensed structural formula for the dinucleotide G C that would be in RNA.
List three structural characteristics of DNA.
Write the base sequence in a complementary DNA segment if each original segment has the following base sequence:
d. C T G T A T A C G T T A
Write the base sequence in a complementary DNA segment if each original segment has the following base sequence:
d. A T A T G C G C T A A A
What is the function of the enzyme helicase in DNA replication?