Textbook Question
Consider the following bonds: Ca and O, C and O, K and O, O and O, and N and O.
d. Arrange the covalent bonds in order of decreasing polarity.
1543
views

 Verified step by step guidance
Verified step by step guidance


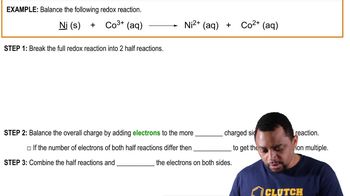
Consider the following bonds: Ca and O, C and O, K and O, O and O, and N and O.
d. Arrange the covalent bonds in order of decreasing polarity.
Consider an ion with the symbol X2+ formed from a representative element.
b. What is the Lewis symbol of the element?
Consider an ion with the symbol X2+ formed from a representative element.
c. If X is in Period 3, what is the element?
Consider an ion with the symbol Y3- formed from a representative element.
b. What is the Lewis symbol of the element?
Consider an ion with the symbol Y3- formed from a representative element.
c. If Y is in Period 3, what is the element?
One of the ions of tin is tin(IV).
a. What is the symbol for this ion?