Textbook Question
Why do chemical reactions require energy of activation?
2470
views

 Verified step by step guidance
Verified step by step guidance
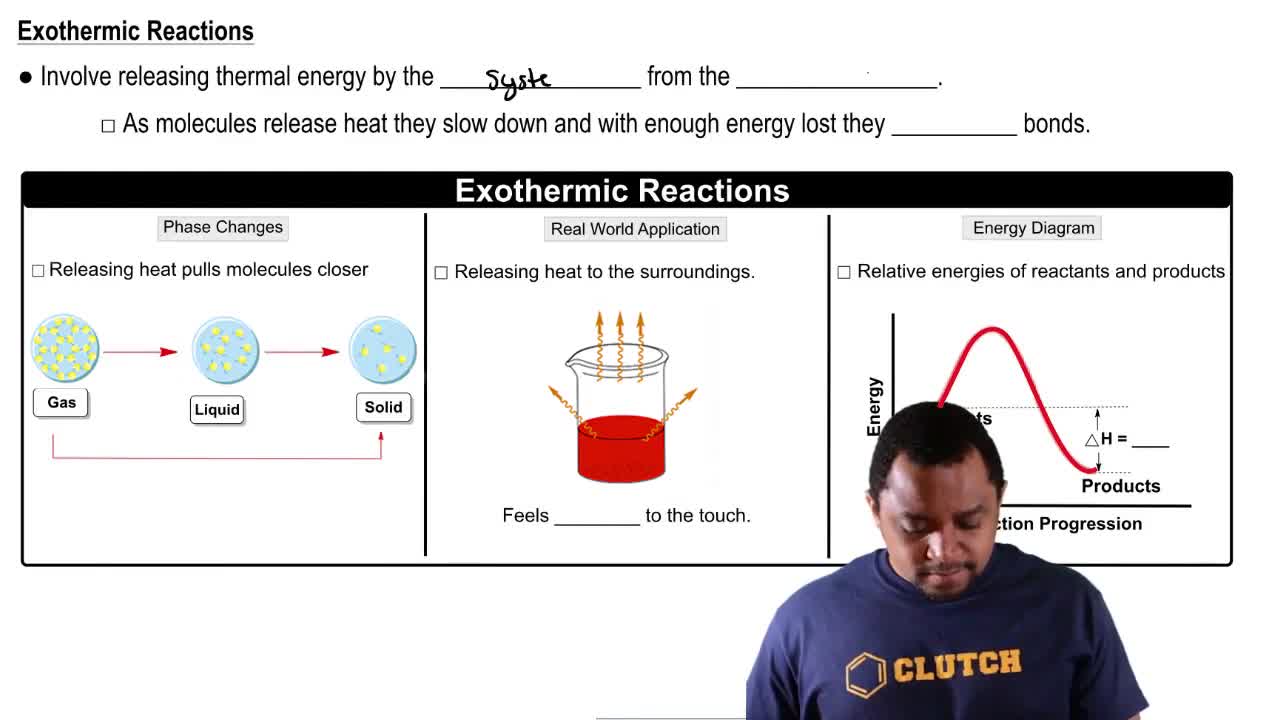
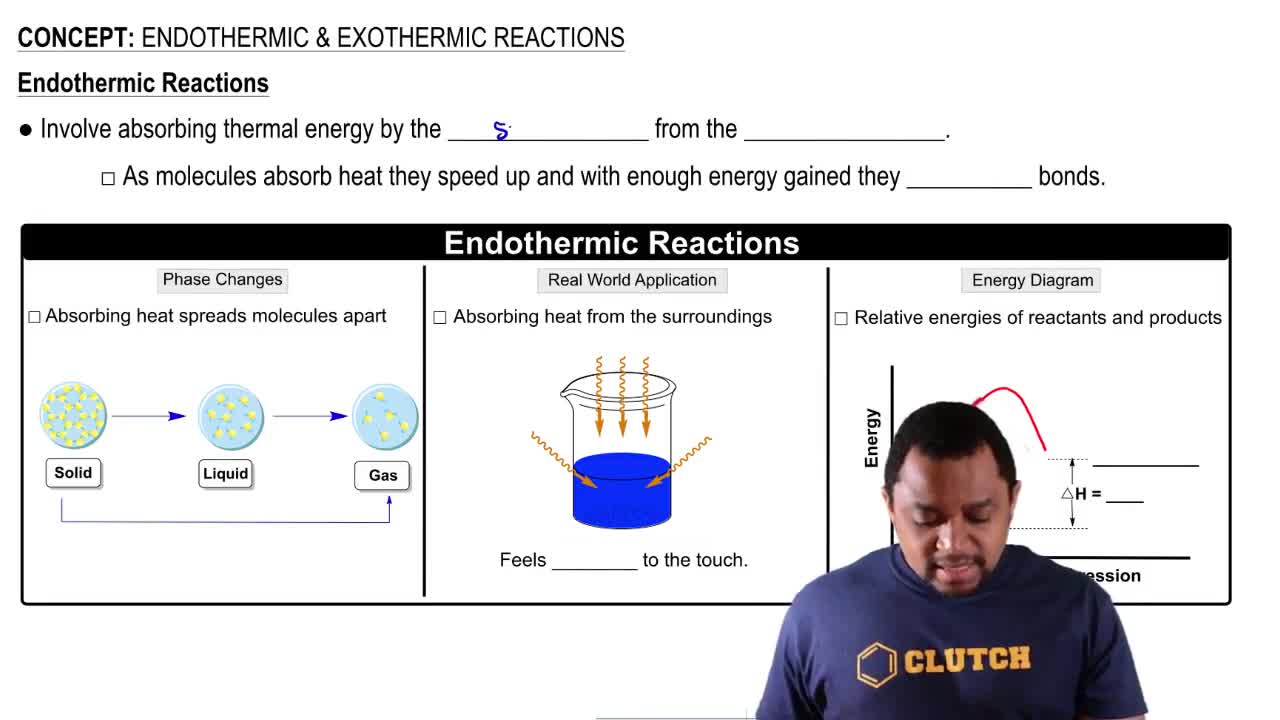

Why do chemical reactions require energy of activation?
Draw an energy diagram for an exothermic reaction.
What is measured by the heat of reaction?
Classify each of the following as exothermic or endothermic:
c. The metabolism of glucose in the body provides energy.
Classify each of the following as exothermic or endothermic:
b. In the body, the synthesis of proteins requires energy.
Classify each of the following as exothermic or endothermic:
a. CH4(g) + 2O2(g) CO2(g) + 2H2O(g) + 802kJ