Textbook Question
What is measured by the heat of reaction?
2374
views

 Verified step by step guidance
Verified step by step guidance
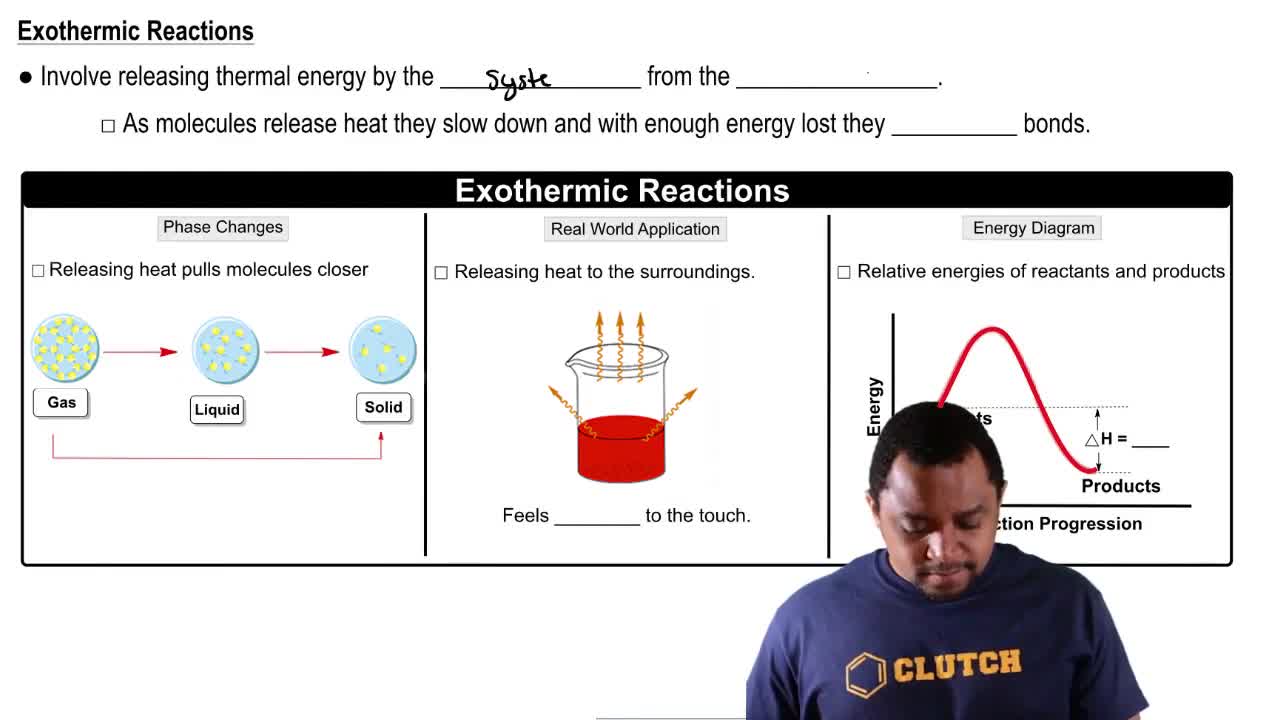
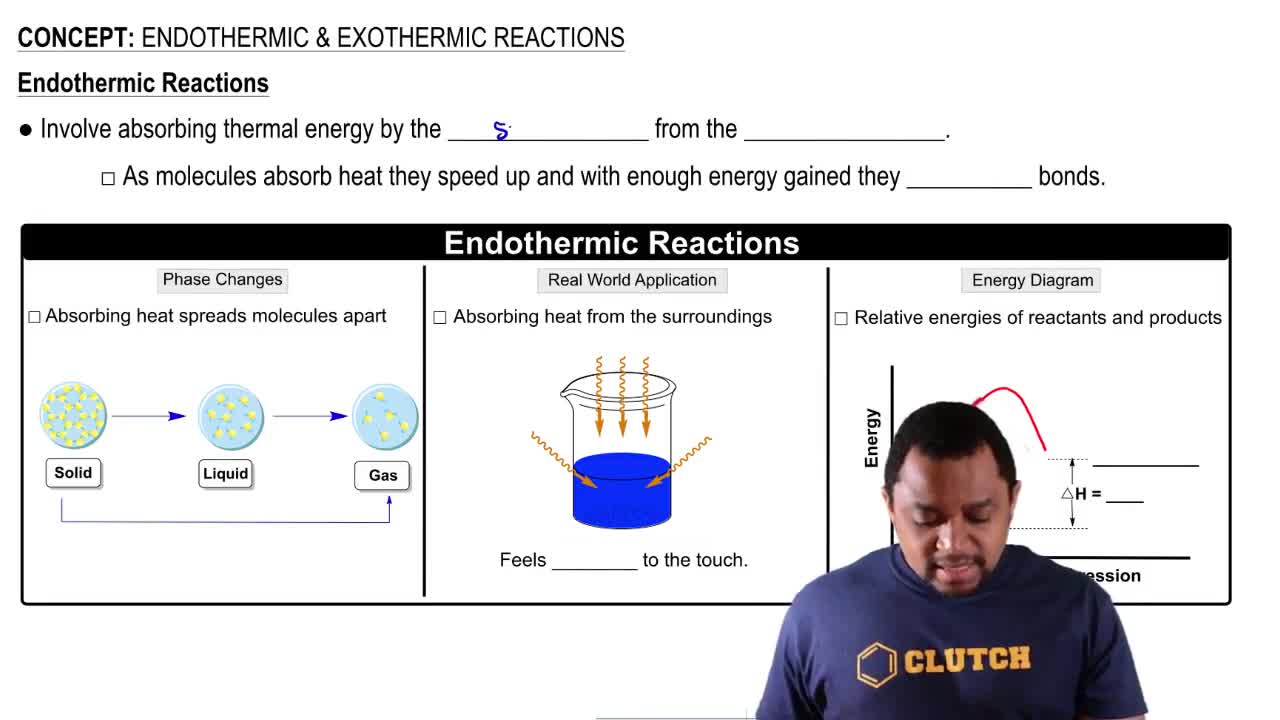

What is measured by the heat of reaction?
Classify each of the following as exothermic or endothermic:
b. The energy level of the products is higher than that of the reactants.
Classify each of the following as exothermic or endothermic:
c. The metabolism of glucose in the body provides energy.
Classify each of the following as exothermic or endothermic:
a. CH4(g) + 2O2(g) CO2(g) + 2H2O(g) + 802kJ
What is meant by the rate of a reaction?
How would each of the following change the rate of the reaction shown here?
2 SO2(g) + O2(g) → 2 SO3(g)
a. adding some SO2(g)