13. Liquids, Solids & Intermolecular Forces
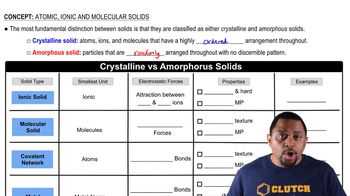
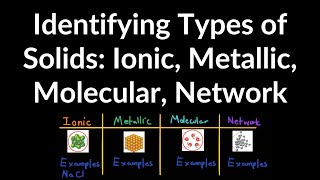
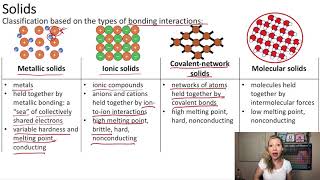
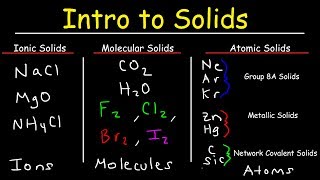
Atomic, Ionic, and Molecular Solids

Get help from an AI Tutor
Ask a question to get started.
Problem 12b
Textbook Question
Textbook QuestionSilicon is the fundamental component of integrated circuits. Si has the same structure as diamond. (b) Silicon readily reacts to form silicon dioxide, SiO2, which is quite hard and is insoluble in water. Is SiO2 most likely a molecular, metallic, ionic, or covalent-network solid?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
50sPlay a video:
719
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos