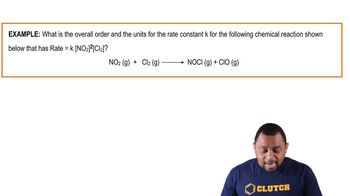
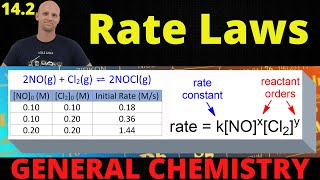
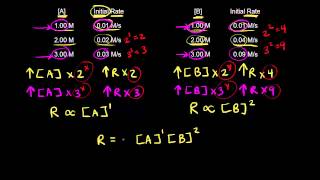15. Chemical Kinetics
Rate Law

Get help from an AI Tutor
Ask a question to get started.
Problem 32a
Textbook Question
Textbook QuestionThe react ion between ethyl bromide 1C2H5Br2 and hydroxide ion in ethyl alcohol at 330 K, C2H5Br1alc2 + OH- 1alc2¡ C2H5OH1l2 + Br - 1alc2, is first order each in ethyl bromide and hydroxide ion. When 3C2H5Br4 is 0.0477 M and 3OH- 4 is 0.100 M, the rate of disappearance of ethyl bromide is 1.7 * 10-7 M>s. (c) How would the rate of disappearance of ethyl bromide change if the solution were diluted by adding an equal volume of pure ethyl alcohol to the solution?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
397
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos