18. Aqueous Equilibrium
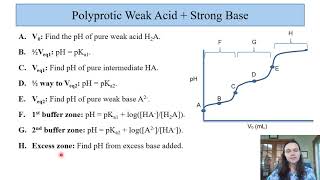
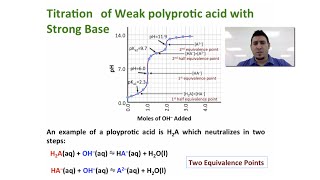
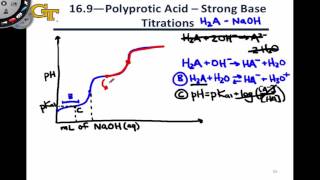
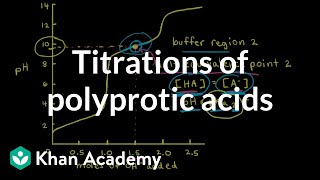
Titrations: Diprotic & Polyprotic Buffers

Get help from an AI Tutor
Ask a question to get started.
Problem 95c
Textbook Question
Textbook QuestionSuppose you want to do a physiological experiment that calls for a pH 6.50 buffer. You find that the organism with which you are working is not sensitive to the weak acid H2A 1Ka1 = 2 * 10-2; Ka2 = 5.0 * 10-72 or its sodium salts. You have available a 1.0 M solution of this acid and a 1.0 M solution of NaOH. How much of the NaOH solution should be added to 1.0 L of the acid to give a buffer at pH 6.50? (Ignore any volume change.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
479
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos