Large amounts of nitrogen gas are used in the manufacture of ammonia, principally for use in fertilizers. Suppose 120.00 kg of N2(g) is stored in a 1100.0-L metal cylinder at 280 °C. (c) Under the conditions of this problem, which correction dominates, the one for finite volume of gas molecules or the one for attractive interactions?

Consider the combustion reaction between 1.00 L of liquid methanol (density = 0.850 g/mL) and 500 L of oxygen gas measured at STP. The products of the reaction are CO2(g) and H2O(g). Calculate the volume of liquid H2O formed if the reaction goes to completion and you condense the water vapor.
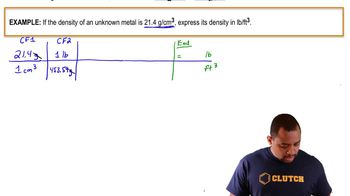
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Stoichiometry

Ideal Gas Law

Density and Volume Conversion
Cyclopropane, a gas used with oxygen as a general anesthetic, is composed of 85.7% C and 14.3% H by mass. (a) If 1.56 g of cyclopropane has a volume of 1.00 L at 99.7 kPa and 50.0 °C, what is the molecular formula of cyclopropane?
Cyclopropane, a gas used with oxygen as a general anesthetic, is composed of 85.7% C and 14.3% H by mass. (b) Judging from its molecular formula, would you expect cyclopropane to deviate more or less than Ar from ideal-gas behavior at moderately high pressures and room temperature? Explain.
An herbicide is found to contain only C, H, N, and Cl. The complete combustion of a 100.0-mg sample of the herbicide in excess oxygen produces 83.16 mL of CO2 and 73.30 mL of H2O vapor expressed at STP. A separate analysis shows that the sample also contains 16.44 mg of Cl. (b) Calculate its empirical formula.
An herbicide is found to contain only C, H, N, and Cl. The complete combustion of a 100.0-mg sample of the herbicide in excess oxygen produces 83.16 mL of CO2 and 73.30 mL of H2O vapor expressed at STP. A separate analysis shows that the sample also contains 16.44 mg of Cl. (c) What other information would you need to know about this compound to calculate its true molecular formula?
A 4.00-g sample of a mixture of CaO and BaO is placed in a 1.00-L vessel containing CO2 gas at a pressure of 97.33 kPa and a temperature of 25 °C. The CO2 reacts with the CaO and BaO, forming CaCO3 and BaCO3. When the reaction is complete, the pressure of the remaining CO2 is 20.0 kPa. (a) Calculate the number of moles of CO2 that have reacted. (b) Calculate the mass percentage of CaO in the mixture.
