The source of oxygen that drives the internal combustion engine in an automobile is air. Air is a mixture of gases, principally N2(79%) and O2(20%). In the cylinder of an automobile engine, nitrogen can react with oxygen to produce nitric oxide gas, NO. As NO is emitted from the tailpipe of the car, it can react with more oxygen to produce nitrogen dioxide gas. (b) Both nitric oxide and nitrogen dioxide are pollutants that can lead to acid rain and global warming; collectively, they are called 'NOx' gases. In 2009, the United States emitted an estimated 19 million tons of nitrogen dioxide into the atmosphere. How many grams of nitrogen dioxide is this?

The thermite reaction, Fe2O3 + Al → Al2O3 + Fe produces so much heat that the Fe product melts. This reaction is used industrially to weld metal parts under water, where a torch cannot be employed. It is also a favorite chemical demonstration in the lecture hall (on a small scale). (c) This reaction produces 852 kJ of heat per mole of Fe2O3 reacted. How many grams of Fe2O3 are needed to produce 1.00 × 104 kJ of heat?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Thermochemical Equations
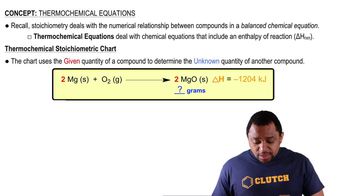
Stoichiometry

Molar Mass

The source of oxygen that drives the internal combustion engine in an automobile is air. Air is a mixture of gases, principally N2(79%) and O2(20%). In the cylinder of an automobile engine, nitrogen can react with oxygen to produce nitric oxide gas, NO. As NO is emitted from the tailpipe of the car, it can react with more oxygen to produce nitrogen dioxide gas. (c) The production of NOx gases is an unwanted side reaction of the main engine combustion process that turns octane, C8H18, into CO2 and water. If 85% of the oxygen in an engine is used to combust octane and the remainder used to produce nitrogen dioxide, calculate how many grams of nitrogen dioxide would be produced during the combustion of 500 g of octane.
The thermite reaction, Fe2O3 + Al → Al2O3 + Fe produces so much heat that the Fe product melts. This reaction is used industrially to weld metal parts under water, where a torch cannot be employed. It is also a favorite chemical demonstration in the lecture hall (on a small scale). (b) Calculate how many grams of aluminum are needed to completely react with 500.0 g of Fe2O3 in this reaction.
The thermite reaction, Fe2O3 + Al S Al2O3 + Fe produces so much heat that the Fe product melts. This reaction is used industrially to weld metal parts under water, where a torch cannot be employed. It is also a favorite chemical demonstration in the lecture hall (on a small scale). (d) If you performed the reverse reaction— aluminum oxide plus iron makes iron oxide plus aluminum—would that reaction have heat as a reactant or a product?
