A sample of 8.69 g of Zn(OH)2 is added to 155.0 mL of 0.750 M H2SO4. (b) Which is the limiting reactant in the reaction?

Ritalin is the trade name of a drug, methylphenidate, used to treat attention-deficit/hyperactivity disorder in young adults. The chemical structure of methylphenidate is (c) Ritalin has a half-life of 3 hours in the blood, which means that after 3 hours the concentration in the blood has decreased by half of its initial value. For the man in part (b), what is the concentration of Ritalin in his blood after 6 hours?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Half-life
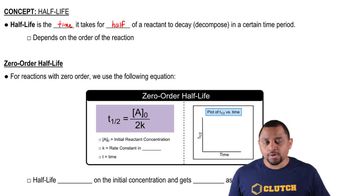
Exponential Decay

Concentration Calculation

A sample of 8.69 g of Zn(OH)2 is added to 155.0 mL of 0.750 M H2SO4. (c) How many moles of Zn(OH)2, H2SO4, ZnSO4 are present after the reaction is complete?
In 2014, a major chemical leak at a facility in West Virginia released 28,390 L of MCHM (4-methylcyclohexylmethanol, C8H16O) into the Elk River. The density of MCHM is 0.9074 g/mL. (a) Calculate the initial molarity of MCHM in the river, assuming that the first part of the river is 2.00 m deep, 90.0 m wide, and 90.0 m long.
The arsenic in a 1.22-g sample of a pesticide was converted to AsO43- by suitable chemical treatment. It was then titrated using Ag+ to form Ag3AsO4 as a precipitate. (a) What is the oxidation state of As in AsO43-?
The arsenic in a 1.22-g sample of a pesticide was converted to AsO43- by suitable chemical treatment. It was then titrated using Ag+ to form Ag3AsO4 as a precipitate. (b) Name Ag3AsO4 by analogy to the corresponding compound containing phosphorus in place of arsenic.
