Textbook Question
Consider the reaction 2 H2(g) + O2(g) → 2 H2O(l). (a) Use the bond enthalpies in Table 5.4 to estimate H for this reaction, ignoring the fact that water is in the liquid state.
500
views

 Verified step by step guidance
Verified step by step guidance
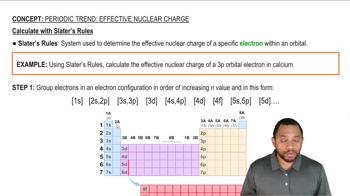


Consider the reaction 2 H2(g) + O2(g) → 2 H2O(l). (a) Use the bond enthalpies in Table 5.4 to estimate H for this reaction, ignoring the fact that water is in the liquid state.
(a) A serving of a particular ready-to-serve brown & wild rice meal contains 4.5 g fat, 42 g carbohydrate, and 4.0 g protein. Estimate the number of calories in a serving.