Textbook Question
Detailed calculations show that the value of Zeff for the outermost electrons in Na and K atoms is 2.51+ and 3.49+, respectively. (b) What values do you estimate for Zeff using Slater’s rules?

 Verified step by step guidance
Verified step by step guidance


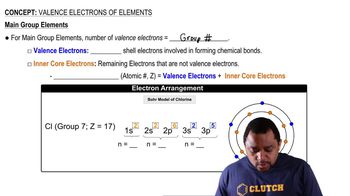
Detailed calculations show that the value of Zeff for the outermost electrons in Na and K atoms is 2.51+ and 3.49+, respectively. (b) What values do you estimate for Zeff using Slater’s rules?
Detailed calculations show that the value of Zeff for the outermost electrons in Na and K atoms is 2.51+ and 3.49+, respectively. (e) Predict Zeff for the outermost electrons in the Rb atom based on the calculations for Na and K.