Assume that you have a cylinder with a movable piston. What would happen to the gas pressure inside the cylinder if you were to do the following? (c) Decrease the volume by 45% at constant T
Ch.10 - Gases: Their Properties & Behavior

Chapter 10, Problem 49a
Assume that you have a cylinder with a movable piston. What would happen to the gas volume of the cylinder if you were to do the following? (a) Halve the Kelvin temperature while holding the pressure constant
 Verified step by step guidance
Verified step by step guidance1
Identify the initial conditions and the changes applied to the system. In this case, the initial condition is the gas in the cylinder at a certain temperature and pressure, and the change is halving the Kelvin temperature while keeping the pressure constant.
Recall the ideal gas law, which is expressed as PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature in Kelvin.
Understand that since the pressure (P) and the number of moles of gas (n) are held constant, and R is a constant, the relationship between volume (V) and temperature (T) can be simplified to V \propto T. This means that the volume is directly proportional to the temperature.
Apply the direct proportionality to predict the effect of the temperature change. If the temperature is halved (T becomes T/2), then the volume will also be halved (V becomes V/2) to maintain the equation V \propto T.
Conclude that when the Kelvin temperature of the gas is halved while keeping the pressure constant, the volume of the gas in the cylinder will also be halved.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law relates the pressure, volume, temperature, and number of moles of a gas through the equation PV = nRT. This law is fundamental in understanding how gases behave under various conditions, allowing predictions about changes in volume when temperature or pressure is altered.
Recommended video:
Guided course

Ideal Gas Law Formula
Charles's Law
Charles's Law states that the volume of a gas is directly proportional to its absolute temperature (in Kelvin) when pressure is held constant. This means that if the temperature decreases, the volume will also decrease, which is crucial for analyzing the scenario of halving the temperature.
Recommended video:
Guided course
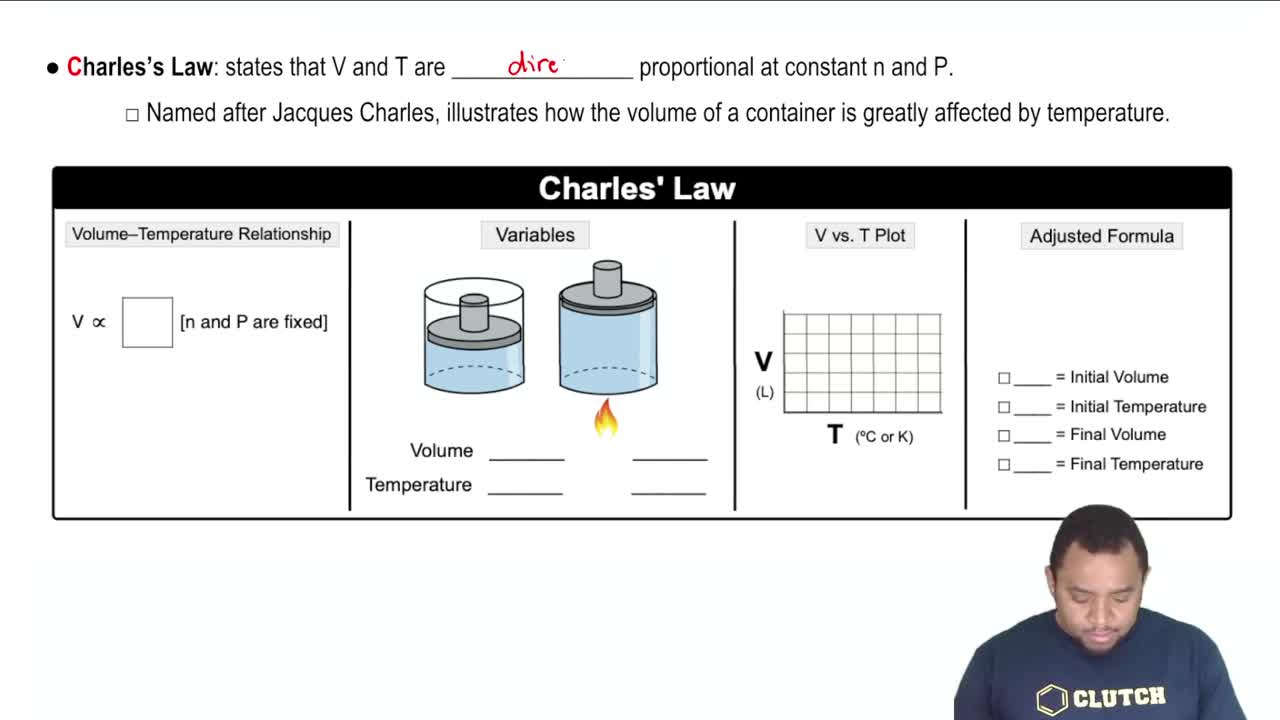
Charles's Law
Absolute Temperature
Absolute temperature is measured in Kelvin and is essential for gas law calculations. It starts at absolute zero, where molecular motion ceases. Understanding that temperature must be in Kelvin is vital when applying gas laws, as using Celsius or Fahrenheit would yield incorrect results.
Recommended video:
Guided course

Temperature vs Heat
Related Practice
Textbook Question
682
views
Textbook Question
Assume that you have a cylinder with a movable piston. What would happen to the gas pressure inside the cylinder if you were to do the following? (d) Halve the Kelvin temperature and triple the volume
466
views
Textbook Question
Assume that you have a cylinder with a movable piston. What would happen to the gas volume of the cylinder if you were to do the following? (b) Increase the amount of gas by one-fourth while holding the temperature and pressure constant
513
views
Textbook Question
Assume that you have a cylinder with a movable piston. What would happen to the gas volume of the cylinder if you were to do the following? (d) Double the Kelvin temperature and double the pressure
967
views
Textbook Question
Which sample contains more molecules: 1.00 L of O2 at STP, 1.00 L of air at STP, or 1.00 L of H2 at STP?
983
views
