In 1911, the groundbreaking gold foil experiment conducted by Ernest Rutherford, along with Hans Geiger and Ernest Marsden, revealed the existence of the positively charged nucleus within an atom. This experiment is often referred to interchangeably as the Rutherford gold experiment or the Geiger-Marsden experiment, highlighting the significant contributions of all three scientists.
The experimental setup involved bombarding a thin sheet of gold foil with alpha particles emitted from a radioactive source, typically iridium, which was encased in a lead box with an opening. An alpha particle consists of 2 protons and 2 neutrons, giving it an atomic number of 2 and a mass number of 4. This can be represented as the elemental symbol \({}^{4}_{2}\text{He}^{2+}\), indicating its positive charge due to the presence of protons.
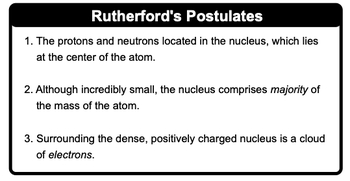
As the alpha particles were directed at the gold foil, the detecting screen surrounding the foil recorded their behavior. Most alpha particles passed through the foil, but some were deflected at various angles, and a few even bounced back toward the source. This unexpected result led Rutherford to formulate three key postulates about atomic structure:
- The nucleus, containing protons and neutrons, is located at the center of the atom.
- Despite its small size, the nucleus accounts for most of the atom's mass.
- A cloud of electrons surrounds the dense, positively charged nucleus.
These postulates fundamentally altered the understanding of atomic structure at the time, challenging previously accepted notions and laying the groundwork for modern atomic theory. The insights gained from the gold foil experiment have since become foundational concepts in chemistry, enhancing our comprehension of atomic composition and behavior.