Textbook Question
Molybdenum (mp 2623 °C) has a higher melting point than yttrium (mp 1522 °C) or cadmium (mp 321 °C). Explain.
115
views

 Verified step by step guidance
Verified step by step guidance

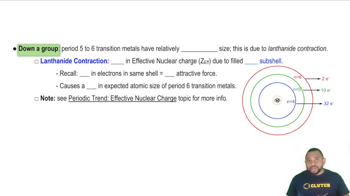
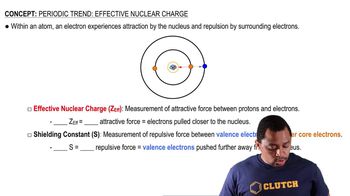
Molybdenum (mp 2623 °C) has a higher melting point than yttrium (mp 1522 °C) or cadmium (mp 321 °C). Explain.
Briefly account for each of the following observations:
(a) Atomic radii decrease in the order Sc > Ti > V.
(b) Densities increase in the order Ti > V > Cr.
Arrange the following atoms in order of decreasing atomic radius, and account for the trend.
(a) Cr
(b) Ti
(c) Mn
(d) V
The atomic radii of zirconium (160 pm) and hafnium (159 pm) are nearly identical. Explain.