Why is Tₘ related to base composition?

A genetics student was asked to draw the chemical structure of an adenine- and thymine-containing dinucleotide derived from DNA. The answer is shown here:
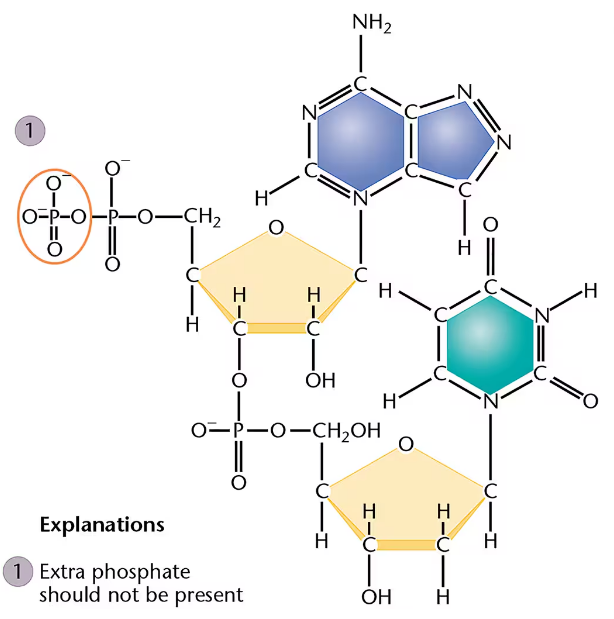
The student made more than six major errors. One of them is circled, numbered 1, and explained. Find five others. Circle them, number them 2 through 6, and briefly explain each in the manner of the example given.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Nucleotide Structure

Phosphodiester Bond Formation
Base Pairing and Orientation

What is the chemical basis of molecular hybridization?
What did the Watson–Crick model suggest about the replication of DNA?
Considering the information on B- and Z-DNA and right- and left-handed helices, carefully analyze structures (a) and (b) below and draw conclusions about their helical nature. Which is right-handed and which is left-handed?
One of the most common spontaneous lesions that occurs in DNA under physiological conditions is the hydrolysis of the amino group of cytosine, converting the cytosine to uracil. What would be the effect on DNA structure of a uracil group replacing cytosine?
In some organisms, cytosine is methylated at carbon 5 of the pyrimidine ring after it is incorporated into DNA. If a 5-methyl cytosine molecule is then hydrolyzed, what base will be generated?
