Consider the following Lewis symbols for elements X and Y:
g. Is the compound in part f ionic or molecular?

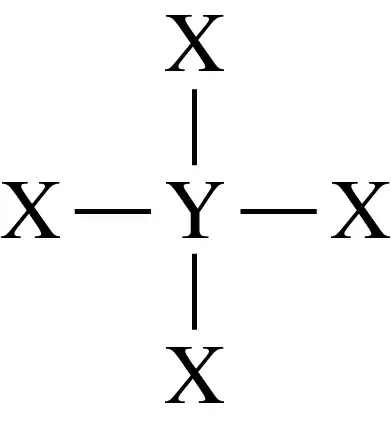
 Verified step by step guidance
Verified step by step guidance


Consider the following Lewis symbols for elements X and Y:
g. Is the compound in part f ionic or molecular?
Using each of the following electron arrangements, give the formulas for the cation and anion that form, the formula for the compound they form, and its name.
State the number of valence electrons, bonding pairs, and lone pairs in each of the following Lewis structures:
c.
Match each of the formulas (a to c) with the correct diagram (1 to 3) of its shape, and name the shape; indicate if each molecule is polar or nonpolar.
<IMAGE>
a. PBr3
Consider the following bonds: Ca and O, C and O, K and O, O and O, and N and O.
d. Arrange the covalent bonds in order of decreasing polarity.
Consider an ion with the symbol X2+ formed from a representative element.
b. What is the Lewis symbol of the element?