Textbook Question
Methyl benzoate, which smells like pineapple guava, is used to train detection dogs.
b. Write the IUPAC name for the carboxylic acid and alcohol used to prepare methyl benzoate.
<IMAGE>
500
views

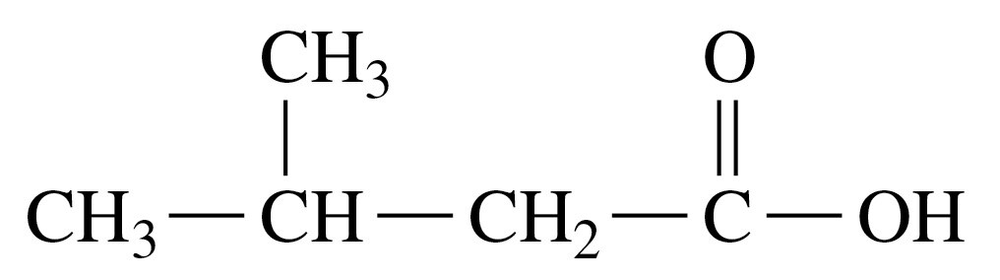
 Verified step by step guidance
Verified step by step guidance


Methyl benzoate, which smells like pineapple guava, is used to train detection dogs.
b. Write the IUPAC name for the carboxylic acid and alcohol used to prepare methyl benzoate.
<IMAGE>
There are four amine isomers with the molecular formula C3H9N. Draw their condensed structural formulas, write the common name, and classify each as a primary (1°), secondary (2°), or tertiary (3°) amine.
There are four amide isomers with the molecular formula C3H7NO. Draw their condensed structural formulas and write the IUPAC name for each.
Write the IUPAC and common names, if any, for each of the following:
c.
Write the IUPAC and common names, if any, for each of the following:
c.
Write the IUPAC and common names, if any, for each of the following:
e.