Textbook Question
Draw the Fischer projection for the other enantiomer of c to d in problem 13.21.
c.
d.
615
views

 Verified step by step guidance
Verified step by step guidance

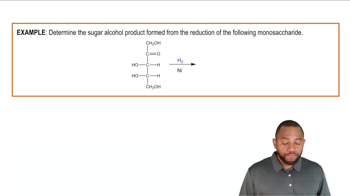

Draw the Fischer projection for the other enantiomer of c to d in problem 13.21.
c.
d.
Draw the Fischer projections for D-glucose and L-glucose.
An infant with galactosemia can utilize D-glucose in milk but not d-galactose. How does the Fischer projection of D-galactose differ from that of D-glucose?
Identify the monosaccharide that fits each of the following descriptions:
a. is also called blood sugar
Identify a monosaccharide that fits each of the following descriptions:
a. found in high blood levels in diabetes
What are the kind and number of atoms in the ring portion of the Haworth structure of glucose?