Textbook Question
Identify the monosaccharide that fits each of the following descriptions:
a. is also called blood sugar
642
views

 Verified step by step guidance
Verified step by step guidance
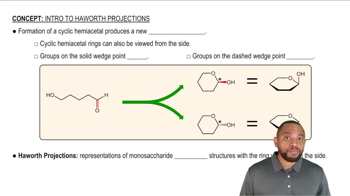
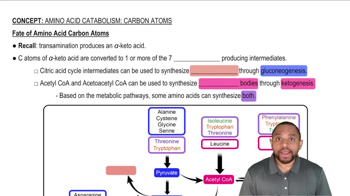
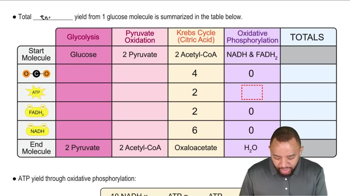
Identify the monosaccharide that fits each of the following descriptions:
a. is also called blood sugar
Identify a monosaccharide that fits each of the following descriptions:
a. found in high blood levels in diabetes
What are the kind and number of atoms in the ring portion of the Haworth structure of glucose?
Identify each of the following as the α or ß isomer:
a.
Draw the Fischer projection for the oxidation and the reduction products of D-xylose. What are the names of the sugar acid and the sugar alcohol produced?
Draw the Fischer projection for the oxidation and the reduction products of D-mannose. What are the names of the sugar acid and the sugar alcohol produced?