Identify each of the following pairs of Fischer projections as enantiomers or identical compounds:
c.

 Verified step by step guidance
Verified step by step guidance

Identify each of the following pairs of Fischer projections as enantiomers or identical compounds:
c.
What are the differences in the Fischer projections of d-fructose and d-galactose?
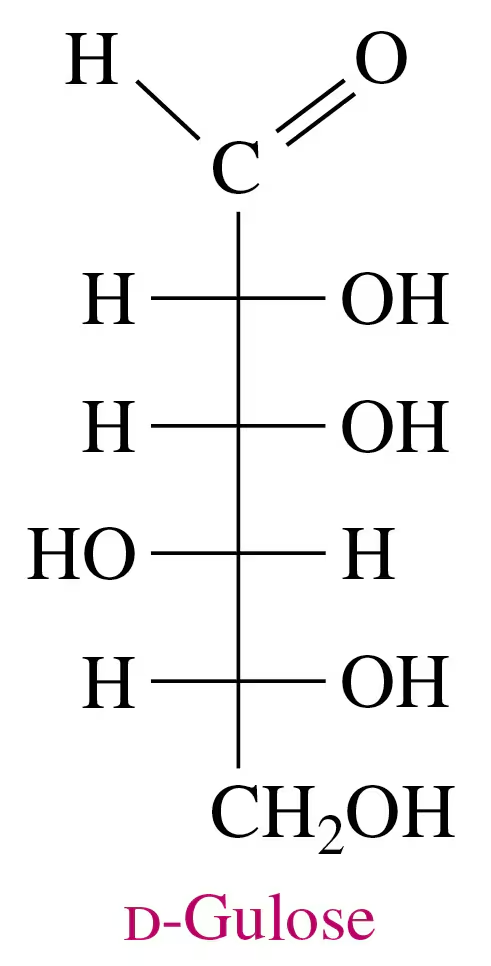
The sugar d-gulose is a sweet-tasting syrup.
a. Draw the Fischer projection for L-gulose.
Use the Fischer projection for d-gulose in problem 13.69 to answer each of the following:
a. Draw the Fischer projection and name the product formed by the reduction of D-gulose.
Use the Fischer projection for D-gulose in problem 13.69 to answer each of the following:
b. Draw the Fischer projection and name the product formed by the oxidation of D-gulose.
D-Erythritol is 70% as sweet as sucrose and contains only hydroxyl functional groups. When d-erythritol is oxidized it forms D-erythrose. Draw the Fischer projection for D-erythritol.