Textbook Question
Write the IUPAC and common name, if any, for each of the following carboxylic acids:
b.
621
views

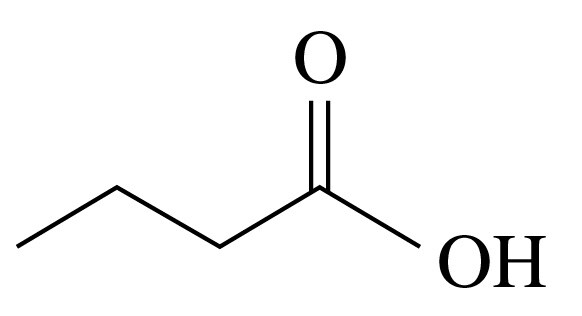
 Verified step by step guidance
Verified step by step guidance


Write the IUPAC and common name, if any, for each of the following carboxylic acids:
b.
Draw the condensed structural formulas for a and b and line-angle formulas for c and d:
a. 3,4-dibromobutanoic acid
Draw the condensed structural formulas for a and b and line-angle formulas for c and d:
c. 3-ethylbenzoic acid