Textbook Question
Identify each of the following bases as a purine or a pyrimidine:
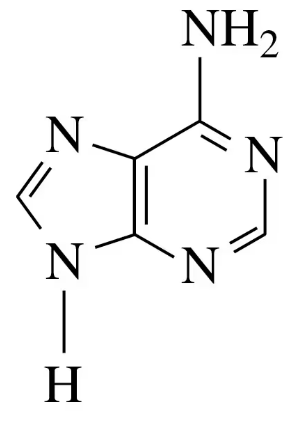
a. guanine
617
views

 Verified step by step guidance
Verified step by step guidance


Identify each of the following bases as a purine or a pyrimidine:
a. guanine
Identify each of the following as a nucleoside or a nucleotide:
b. deoxycytidine
Identify each of the following as a nucleoside or a nucleotide:
d. cytidine monophosphate