Consider the following solids. The solids A, B, and C represent aluminum (D = 2.70g/mL), and silver (D = 10.5 g/mL). If each has a mass of 10.0 g, what is the identity of each solid?
<IMAGE>

 Verified step by step guidance
Verified step by step guidance

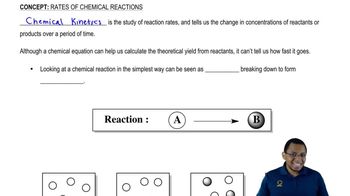

Consider the following solids. The solids A, B, and C represent aluminum (D = 2.70g/mL), and silver (D = 10.5 g/mL). If each has a mass of 10.0 g, what is the identity of each solid?
<IMAGE>
The gray cube has a density of 4.5 g/cm3. Is the density of the green cube the same, lower than, or higher than that of the gray cube?
<IMAGE>
In France, grapes are 1.95 euros per kilogram. What is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro?
The water level in a graduated cylinder initially at 215 mL rises to 285 mL after a piece of lead is submerged. What is the mass, in grams, of the lead?
A graduated cylinder contains 155 mL of water. A 15.0-g piece of iron and a 20.0-g piece of lead are added. What is the new water level, in milliliters, in the cylinder?
How many milliliters of gasoline have a mass of 1.2 kg?