Identify each of the following changes of state as melting, freezing, sublimation, or deposition:
c. Heat is removed from 125 g of liquid water at 0 °C.

 Verified step by step guidance
Verified step by step guidance

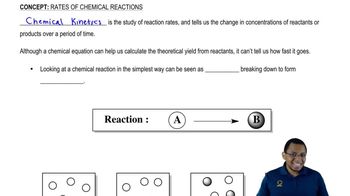
Identify each of the following changes of state as melting, freezing, sublimation, or deposition:
c. Heat is removed from 125 g of liquid water at 0 °C.
Identify each of the following changes of state as melting, freezing, sublimation, or deposition:
d. Frost (ice) forms on the walls of a freezer unit of a refrigerator.
Using the values for the heat of fusion, specific heat of water, and/or heat of vaporization, calculate the amount of heat energy in each of the following:
c. kilojoules needed to melt 24.0 g of ice at 0 °C, warm the liquid to 100 °C, and change it to steam at 100 °C
Using energy values from TABLE 3.8, determine each of the following:
d. If expending 3500 kcal is equal to a loss of 1.0 lb, how many days will it take Charles to lose 5.0 lb?
For the amount of exercise that Charles did for one week in part a, if expending 3500 kcal is equal to a loss of 1.0 lb, how many pounds did he lose?
After a week, biochemical reactions in compost slow, and the temperature drops to 45 °C. The dark brown organic-rich mixture is ready for use in the garden. What is this temperature in degrees Fahrenheit? In kelvins? (3.3)