Textbook Question
Write the names for the elements in each of the following formulas of compounds used in medicine:
c. Demerol, C15H22ClNO2
1316
views

 Verified step by step guidance
Verified step by step guidance
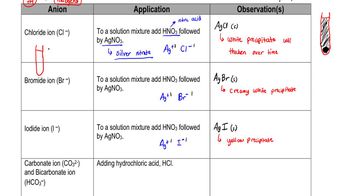

Write the names for the elements in each of the following formulas of compounds used in medicine:
c. Demerol, C15H22ClNO2
Write the names for the elements in each of the following formulas of compounds used in medicine:
a. salt substitute, KCl
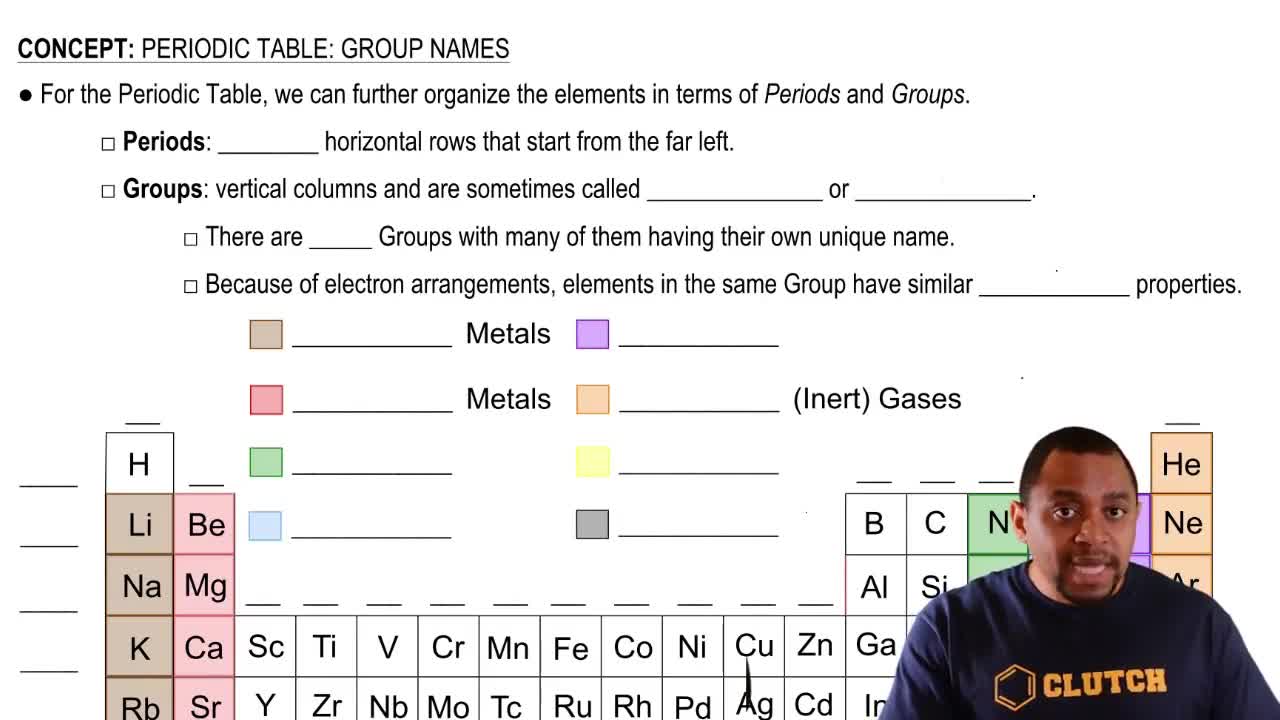
Identify the group or period number described by each of the following:
a. contains C, N, and O
Give the symbol of the element described by each of the following:
a. the alkaline earth metal in Period 2
Identify each of the following elements as a metal, a nonmetal, or a metalloid:
e. located in Group 8A (18)
Identify each of the following elements as a metal, a nonmetal, or a metalloid:
a. located in Group 2A (2)