Textbook Question
Balance each of the following chemical equations:
c. Sb2S3(s) + HCl(aq) → SbCl3(aq) + H2S(g)
2140
views

 Verified step by step guidance
Verified step by step guidance
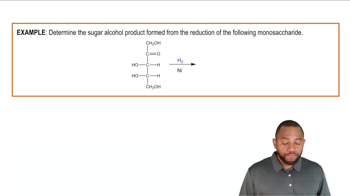

Balance each of the following chemical equations:
c. Sb2S3(s) + HCl(aq) → SbCl3(aq) + H2S(g)
Balance each of the following chemical equations:
d. Al(s) + HCl(aq) → H2(g) + AlCl3(aq)
Identify each of the following as an oxidation or a reduction:
c. Cr3+(aq) + 3e– → Cr(s)
The chemical reaction of hydrogen with oxygen produces water.
2 H2(g) + O2(g) → 2 H2O(g)
c. How many moles of H2O form when 2.5 moles of O2 reacts?
Why do chemical reactions require energy of activation?
Draw an energy diagram for an exothermic reaction.