Select the container that represents the dilution of a 4% (m/v) KCl solution to give each of the following:
a. a 2% (m/v) KCl solution
<IMAGE>

 Verified step by step guidance
Verified step by step guidance
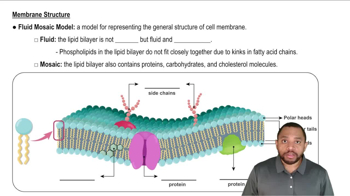
Select the container that represents the dilution of a 4% (m/v) KCl solution to give each of the following:
a. a 2% (m/v) KCl solution
<IMAGE>
A pickle is made by soaking a cucumber in brine, a salt-water solution. What makes the smooth cucumber become wrinkled like a prune?
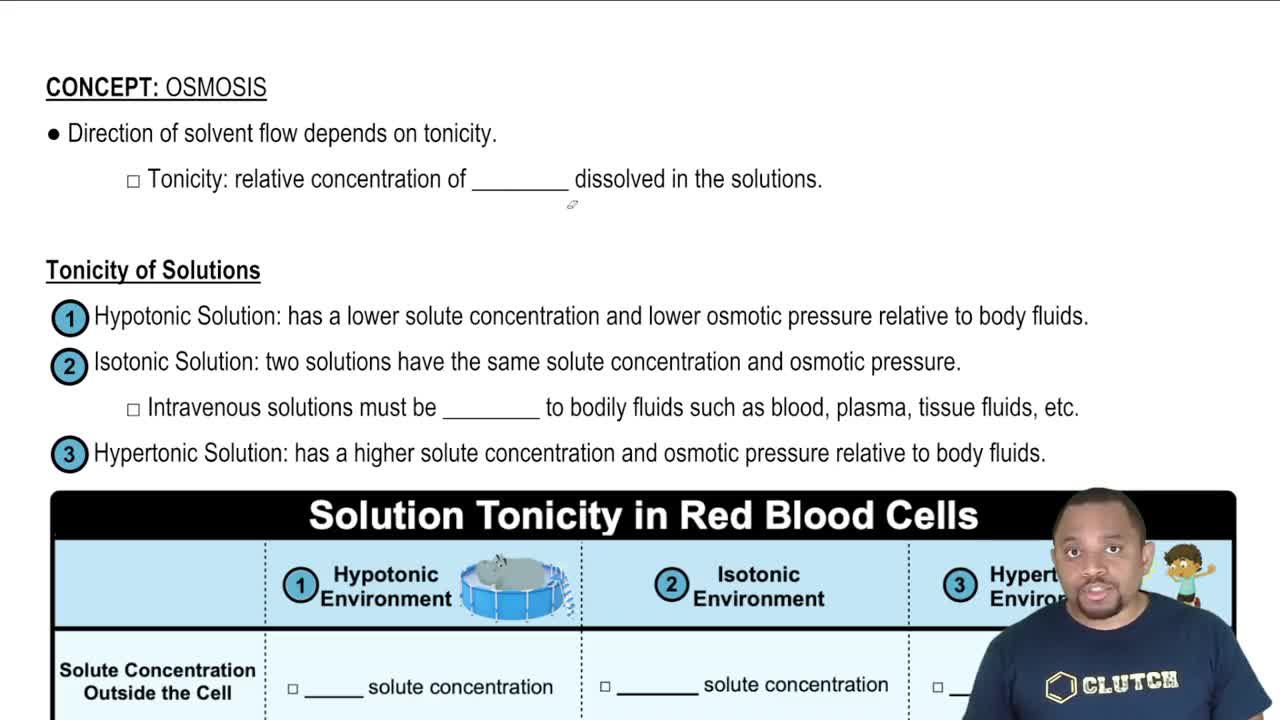
A semipermeable membrane separates two compartments, A and B. If the levels in A and B are equal initially, select the diagram that illustrates the final levels in a to d:
<IMAGE>
Potassium nitrate has a solubility of 32 g of KNO3 in 100. g of H2O at 20 °C. Determine if each of the following forms an unsaturated or saturated solution at 20 °C:
a. adding 32 g of KNO3 to 200. g of H2O
An 80-proof brandy is a 40.% (v/v) ethanol solution. The "proof" is twice the percent concentration of alcohol in the beverage. How many milliliters of alcohol are present in 750 mL of brandy?
In a laboratory experiment, a 10.0-mL sample of NaCl solution is poured into an evaporating dish with a mass of 24.10 g. The combined mass of the evaporating dish and NaCl solution is 36.15 g. After heating, the evaporating dish and dry NaCl have a combined mass of 25.50 g.
a. What is the mass percent (m/m) of the NaCl solution?