When Michelle's blood was tested, the chloride level was 0.45 g/dL.
b. According to TABLE 9.6, is this value above, below, or within the normal range?

 Verified step by step guidance
Verified step by step guidance



When Michelle's blood was tested, the chloride level was 0.45 g/dL.
b. According to TABLE 9.6, is this value above, below, or within the normal range?
State whether each of the following refers to a saturated or an unsaturated solution:
a. A crystal added to a solution does not change in size.
State whether each of the following refers to a saturated or an unsaturated solution:
c. A uric acid concentration of 4.6 mg/100 mL in the kidney does not cause gout.
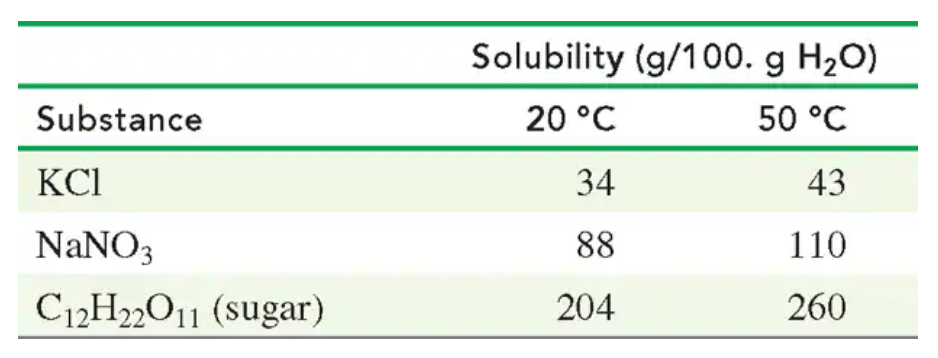
Use the following table:
A solution containing 80. g of NaNO3 in 75 g of H2O at 50 °C is cooled to 20 °C.
b. How many grams of solid NaNO3 crystallized after cooling?
Explain the following observations:
a. More sugar dissolves in hot tea than in iced tea.
Predict whether each of the following ionic compounds is soluble in water:
c. BaCO3