Argon has three naturally occurring isotopes, with mass numbers 36, 38, and 40.
c. How are they different?

 Verified step by step guidance
Verified step by step guidance

Argon has three naturally occurring isotopes, with mass numbers 36, 38, and 40.
c. How are they different?
Argon has three naturally occurring isotopes, with mass numbers 36, 38, and 40.
d. Why is the atomic mass of argon listed on the periodic table not a whole number?
Indium consists of two isotopes, 11349In and 11549In. If the atomic mass for indium on the periodic table is 114.8, are there more atoms of 11349In or 11549In in a sample of indium?
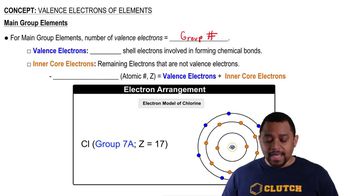
Write the group number and draw the Lewis symbol for each of the following elements:
c. calcium
Write the group number and draw the Lewis symbol for each of the following elements:
e. gallium
Fill in the following blanks using larger or smaller, higher or lower. Mg has a _______ atomic size and a _______ ionization energy than Cs.