An 80-proof brandy is a 40.% (v/v) ethanol solution. The "proof" is twice the percent concentration of alcohol in the beverage. How many milliliters of alcohol are present in 750 mL of brandy?

In a laboratory experiment, a 10.0-mL sample of NaCl solution is poured into an evaporating dish with a mass of 24.10 g. The combined mass of the evaporating dish and NaCl solution is 36.15 g. After heating, the evaporating dish and dry NaCl have a combined mass of 25.50 g.
c. If water is added to 10.0 mL of the initial NaCl solution to give a final volume of 60.0 mL, what is the molarity of the diluted NaCl solution?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Molarity

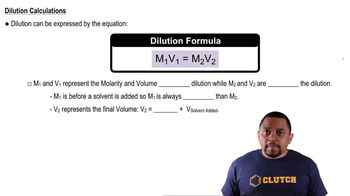
Dilution
Volume Conversion

In a laboratory experiment, a 10.0-mL sample of NaCl solution is poured into an evaporating dish with a mass of 24.10 g. The combined mass of the evaporating dish and NaCl solution is 36.15 g. After heating, the evaporating dish and dry NaCl have a combined mass of 25.50 g.
a. What is the mass percent (m/m) of the NaCl solution?
In a laboratory experiment, a 10.0-mL sample of NaCl solution is poured into an evaporating dish with a mass of 24.10 g. The combined mass of the evaporating dish and NaCl solution is 36.15 g. After heating, the evaporating dish and dry NaCl have a combined mass of 25.50 g.
b. What is the molarity (M) of the NaCl solution?
A solution is prepared with 70.0 g of HNO3 and 130.0 g of H2O. The HNO3 solution has a density of 1.21 g/mL.
b. What is the total volume, in milliliters, of the solution?
A solution is prepared with 70.0 g of HNO3 and 130.0 g of H2O. The HNO3 solution has a density of 1.21 g/mL. (9.4)
d. What is the molarity (M) of the solution?
